PRE-USE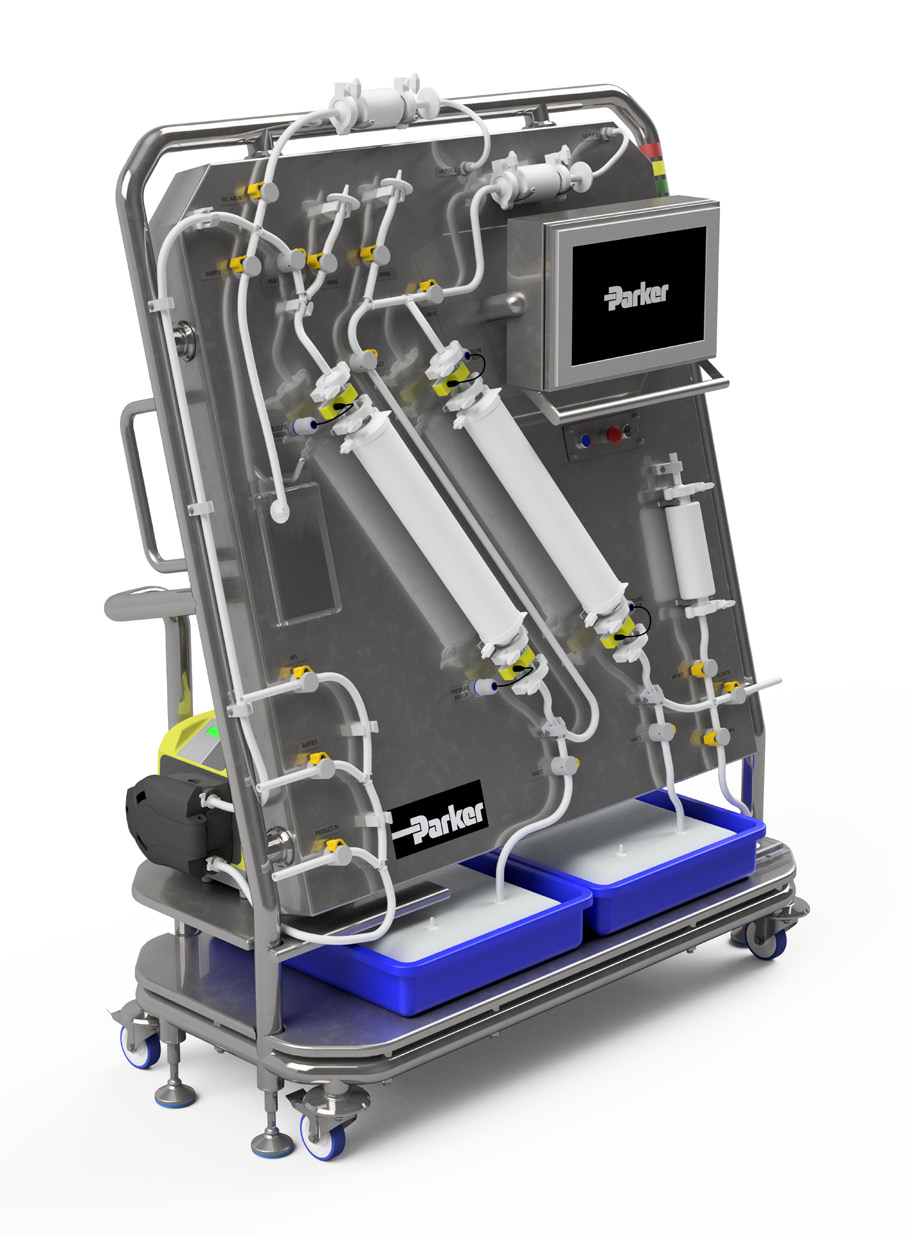
POST STERILIZATION
INTEGRITY TESTING
On the 25th of August 2023, the latest revision of EU GMP Annex 1, Manufacture of Sterile Medicinal Products entered operation.
For manufacturers who cannot terminally sterilize their product in its final container, and therefore need to employ sterile filtration, the revised Annex 1 states Pre-Use Post-Sterilization Integrity Testing (PUPSIT) is now a default requirement.
PUPSIT requires a high degree of planning prior to implementation to ensure the system design and operation is safe, repeatable, reliable, it does not compromise product sterility and is not a burden to manufacturing operations.
To implement PUPSIT, the final sterilizing process will need to be assessed, as the system and operations will become more difficult
The following steps can be considered when implementing your PUPSIT system:
- Filter selection
- Filter integrity test method selection
- Wetting method
- System design
- System location
- System operation
| SciLog NFF+ Normal Flow Filtration System with PUPSIT |
